Allergan Eye Drops Recall 2025. The second artificial tears recall was announced in late october 2025, which. Bausch and lomb, which makes soothexp;
Look up any otc eye drop sold in the united states for recalls and red alerts. The second artificial tears recall was announced in late october 2025, which.
Allergan Refresh Tears 15ML CLINIX PHARMACY, If pathogens from contaminated eye drops are applied directly to the eye, the resulting infection could also lead to vision loss and even blindness, the fda warns.

(GWP) Allergan Refresh Tears Eye Drops 15MLBig Pharmacy Malaysia, Food and drug administration (fda) recall list after the agency issued a warning a.

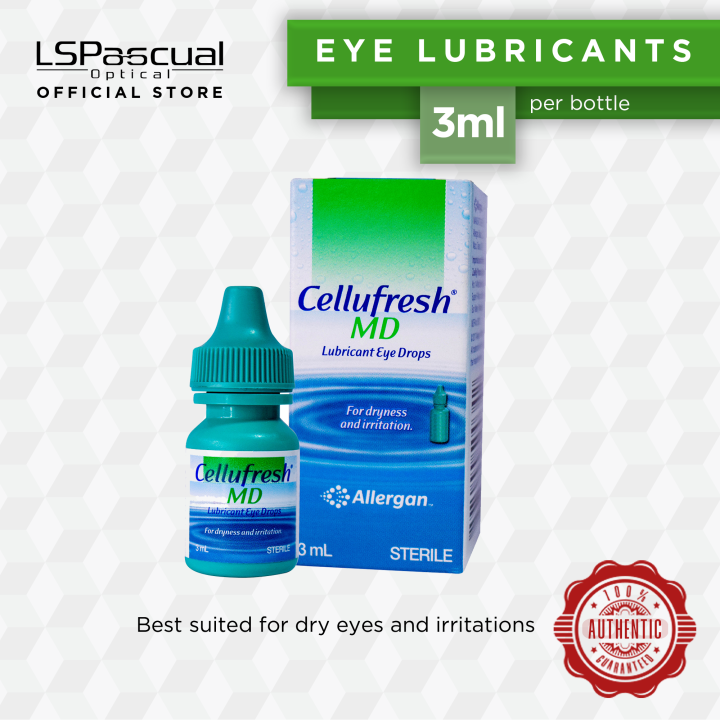
Allergan Cellufresh MD Lubricant Eye Drops EXPIRY 3mL 02/2025, 15mL 06/, The major pharmaceutical company allergan, owned by abbvie, has issued a recall of two eye ointments under the refresh brand that are targeted to treat dry eye.

Allergan Refresh Soothe & Protect Eye Drops Chemist Direct, Food and drug administration (fda) recall list after the agency issued a warning a.
(Expiry 08/2025) REFRESH Tears Eye Drops15mlAllergan/Abbvie Shopee, Food and drug administration recalled another batch of eye drops just weeks after it warned consumers about over two dozen eye drop products.

Refresh Eye Drops Recall 2025 Roana Sheilah, The fda received reports of eye infections, vision loss, and other serious events, including death, that may be linked to using these eye drops.

Refresh Eye Drops Recall 2025 Roana Sheilah, Why are certain brands of eye ointments being recalled?
(6 BOXES) ALLERGAN Refresh TEARS Lubricant Eye Drops 15mL EXP08/2025, “to date, none of the recalled eye drops came from major ophthalmic pharmaceutical firms,” novack said.
